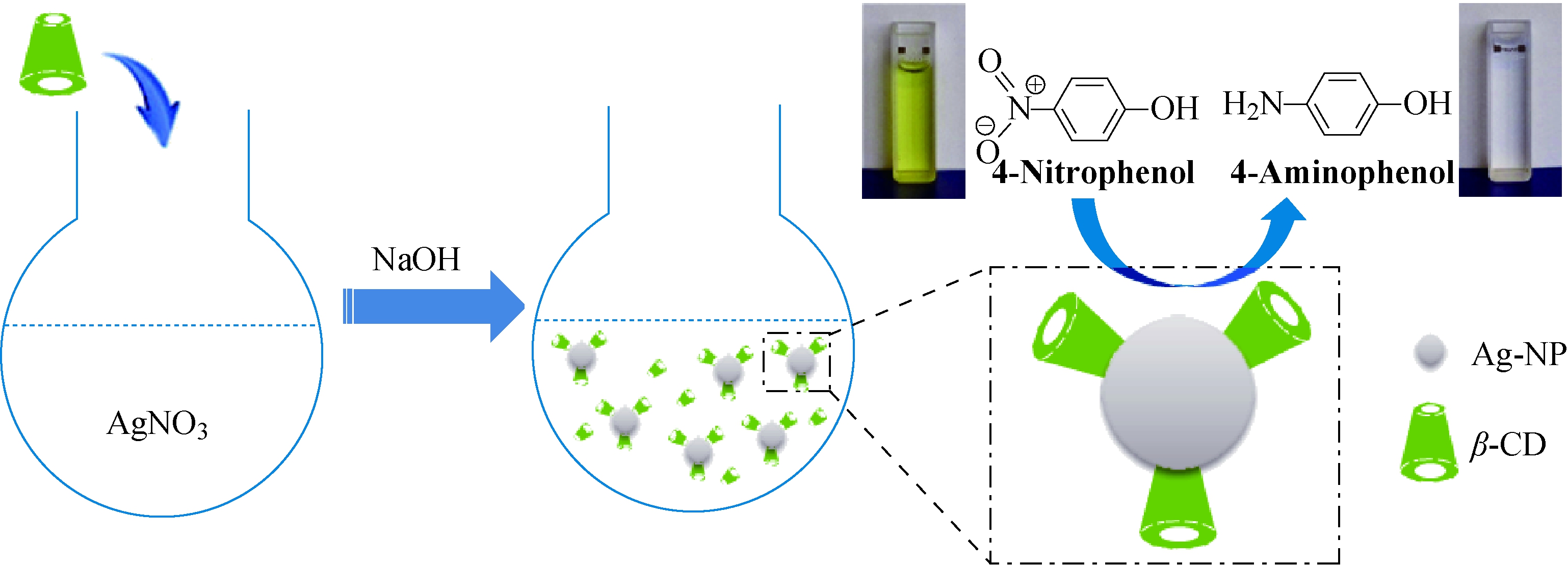
图1 银纳米粒子制备示意图
Fig.1 Schematic illustration of the preparation of Ag-NP
纳米金属颗粒尺寸较小,具有极大的比表面积,表现出与宏观材料显著不同的化学物理性质[1],被广泛应用于催化剂[2]、医学[3]以及电子工业[4]等领域。纳米金属容易发生团聚,影响使用效率[5],所以经常在合成纳米金属时加入修饰剂或载体来阻止其团聚,例如树枝状大分子[6]、微凝胶[7]和聚合物刷[8]等。在各种纳米金属中,纳米银由于在催化[9]、抗菌[3] 和材料修饰[10-11]等方面表现突出,从而成为纳米金属领域的一个研究热点。
值得注意的是,目前研究者开始尝试用更加绿色环保的方法来合成纳米金属。例如文献[12]用葡萄糖作为还原剂,淀粉作为稳定剂,在水溶液中成功合成了银纳米粒子。Liu等[13]利用葡萄糖作为还原剂和稳定剂,在弱酸性环境中制备得到金纳米粒子。环糊精(CD)[14]呈中空圆台型结构, 其内疏水外亲水的特性,可以包裹疏水性有机分子,将许多有机相反应转移到水相中,提高了反应的经济性和环保性。
本文选择环糊精作为还原剂和稳定剂,在弱碱性水溶液中原位还原硝酸银制备得到粒径均一的银纳米粒子,并探究其催化活性。
1 实验部分
1.1 实验原料及仪器
硝酸银,分析纯,国药集团化学试剂有限公司;α/β/γ-环糊精,分析纯,国药集团化学试剂有限公司;对硝基苯酚,分析纯,上海凌峰化学试剂有限公司;氢氧化钠,分析纯,上海凌峰化学试剂有限公司;硼氢化钠,分析纯,上海天莲化工科技有限公司;去离子水,实验室自制。
高倍透射显微镜(HR-TEM),JEOL 2100F;紫外分光光度计,岛津UV2550;傅里叶红外光谱仪(FT-IR),Bruker Vertex 70;Zeta电位仪,NICOMPTM 380 ZLS型。
1.2 银纳米粒子的制备
环糊精包覆的银纳米粒子的制备过程如图1所示,在100 mL三口烧瓶中,加入去离子水29 mL、5 mmol/L 环糊精溶液20 mL、10 mmol/L硝酸银溶液1 mL,并用磁力搅拌器搅拌混合均匀。然后用1 mol/L氢氧化钠溶液调节溶液pH,在60 ℃下反应2 h,制备得到银纳米粒子(Silver Nanoparticles,Ag-NP)。
2 结果与讨论
2.1 环糊精浓度对银纳米粒子粒径的影响
分别取不同浓度的β-环糊精制备得到不同的银纳米粒子,其UV-vis吸收光谱如图2所示。从图2可以看出,环糊精浓度较低时,生成的银纳米粒子的UV-vis吸收峰红移很明显,且吸收峰变宽,意味着合成的银纳米粒子的粒径变大。这是由于环糊精起到还原剂和稳定剂的作用,当环糊精浓度升高时,环糊精还原硝酸银生成的纳米金属银的成核点变多,再加上环糊精作为稳定剂,也阻止了银纳米粒子的靠拢和聚并。
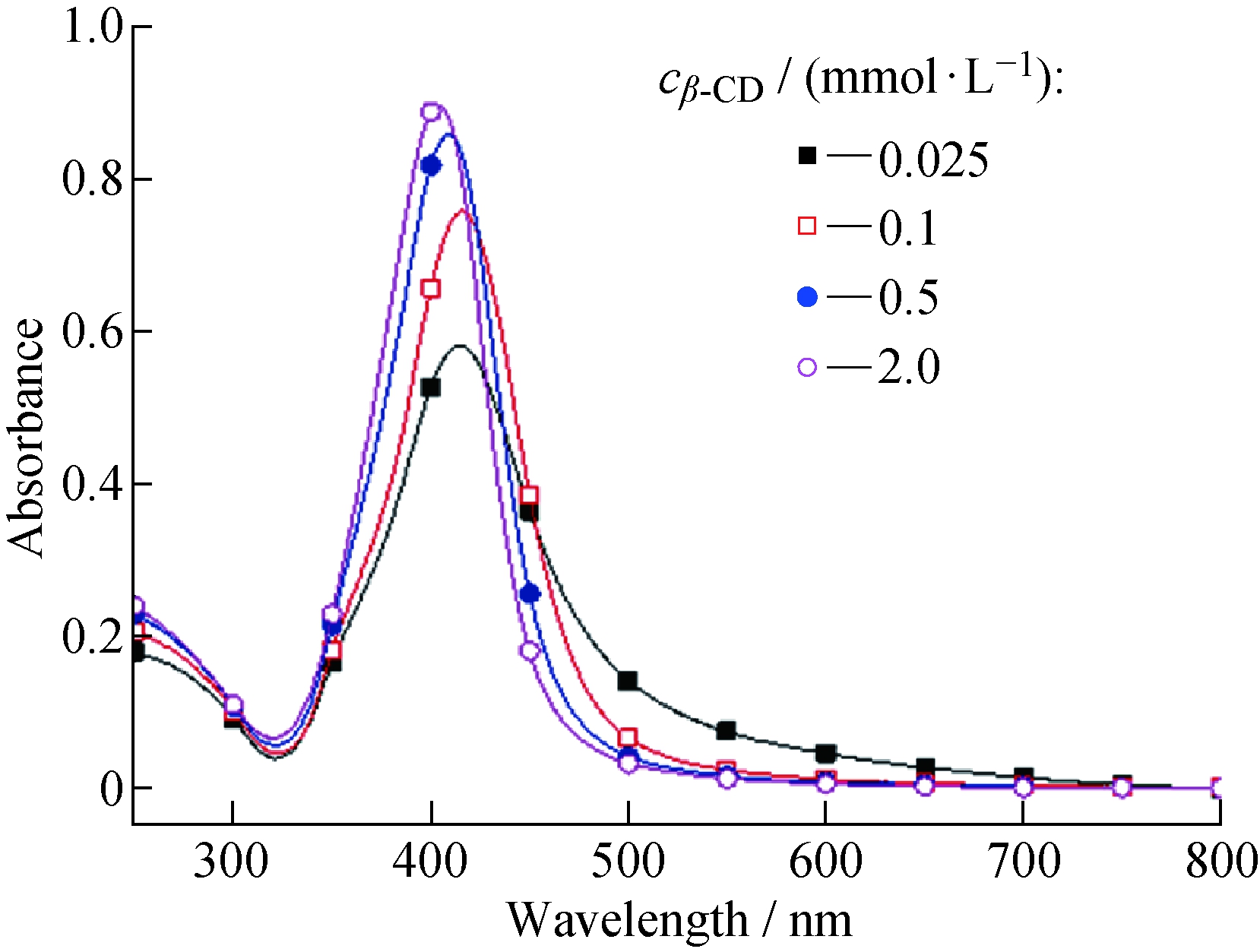
图2 不同浓度β-CD制得的银纳米粒子的UV-vis吸收光谱(pH=10.19)
Fig.2 UV-visible absorption spectra of Ag-NP prepared by different concentrations of β-CD (pH=10.19)
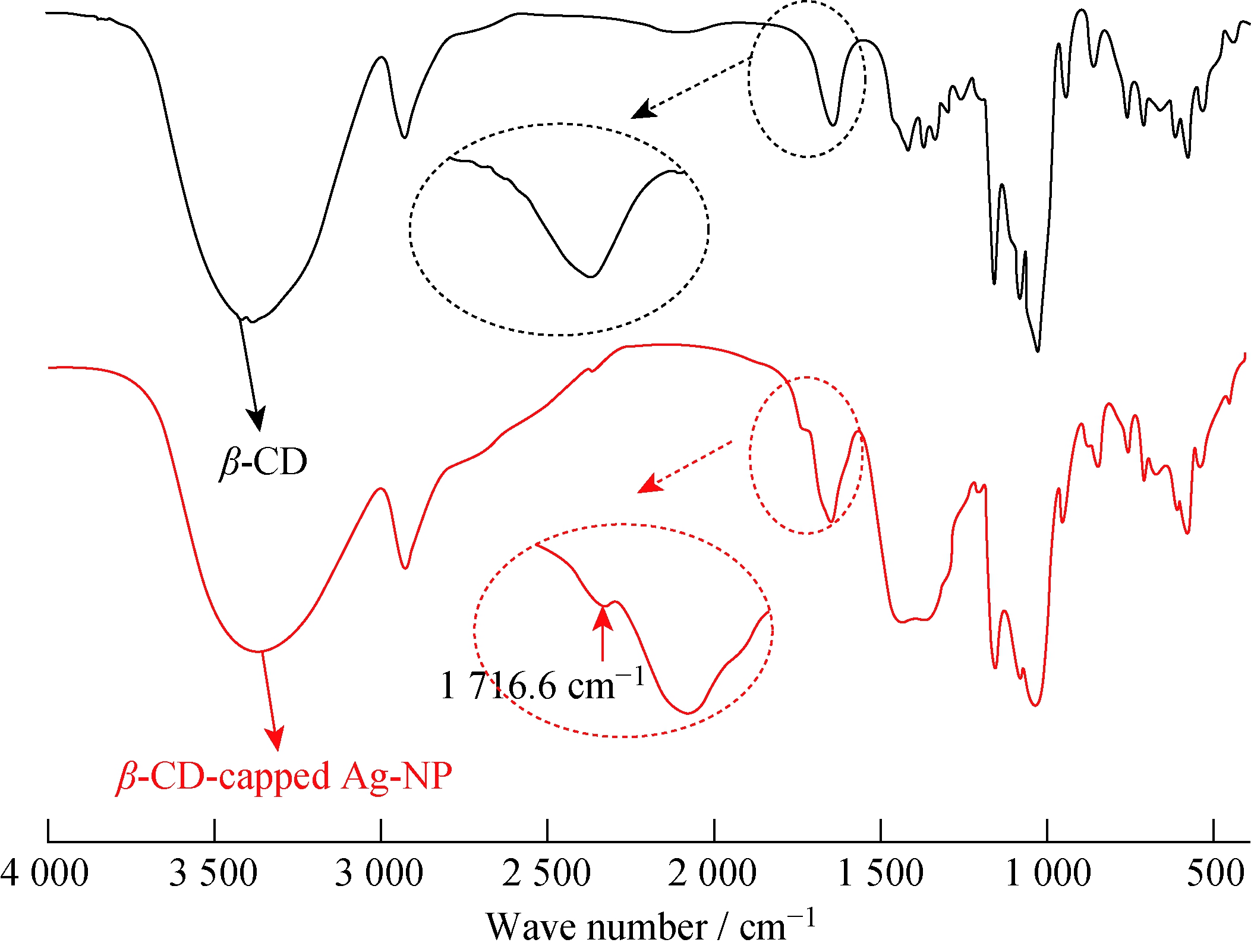
图4 包覆前后的β-环糊精红外光谱图
Fig.4 FT-IR spectra of free β-CD and β-CD-capped Ag-NP
2.2 pH对银纳米粒子粒径的影响
pH在银纳米粒子的形成过程中起着重要的作用,不同pH下制得银纳米粒子的UV-vis吸收光谱如图3所示。从图3可以看到,在酸性环境下(pH<7),即使延长反应时间,溶液中也没有银纳米粒子生成(紫外光谱为一条直线);而在碱性环境下(pH>7),反应 2 h,紫外光谱图中有明显的银纳米粒子的特征吸收峰。这是由于碱性环境有利于环糊精上羟基的质子化,提高了环糊精的还原能力[15]。实验还发现,溶液碱性太强(pH > 12)会导致银纳米粒子团聚,原因可能是环糊精上羟基质子化过多,静电作用不能很好包覆在银纳米粒子表面。
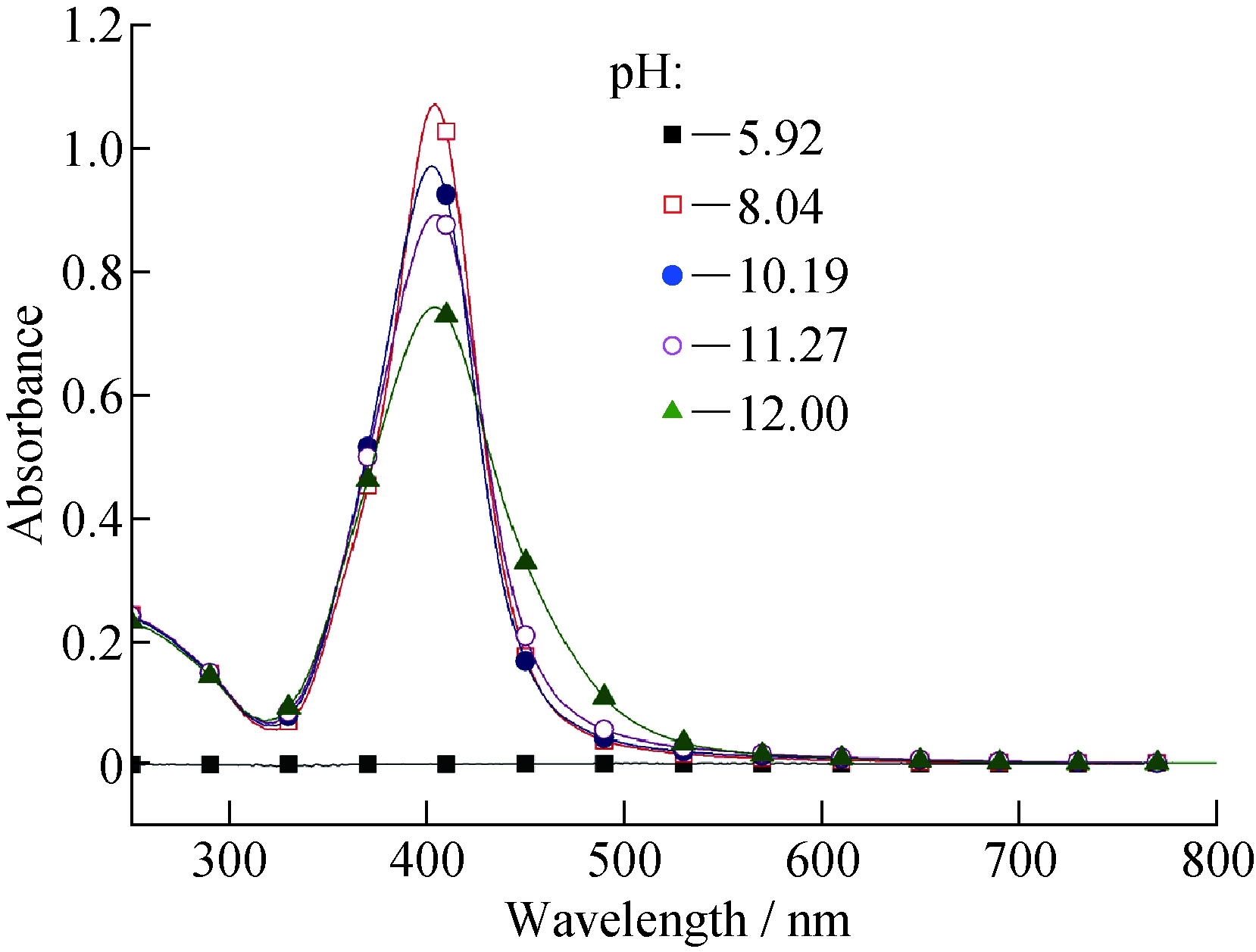
图3 不同pH下制得银纳米粒子的UV-vis吸收光谱(cβ-CD=2.0 mmol/L)
Fig.3 UV-visible absorption spectra of Ag-NP at different pH (cβ-CD=2.0 mmol/L)
2.3 环糊精包覆的银纳米粒子的红外光谱和TEM
在银纳米粒子的制备中,环糊精起到了还原剂和稳定剂的作用。图4示出了包覆前后的β-环糊精红外光谱图。从图4可以看出,1 716.6 cm-1处的新增峰为C![]() O的伸缩振动吸收峰,这是由于环糊精上的羟基将溶液中的Ag+还原成Ag-NP,自身被氧化成羧基[15]。环糊精包覆的银纳米粒子的Zeta电位值稳定在-40 mV左右,说明还原反应后,环糊精上的羧基能和银纳米粒子形成很好的Ag-COO- 相互作用,更有利于稳定银纳米粒子[15]。
O的伸缩振动吸收峰,这是由于环糊精上的羟基将溶液中的Ag+还原成Ag-NP,自身被氧化成羧基[15]。环糊精包覆的银纳米粒子的Zeta电位值稳定在-40 mV左右,说明还原反应后,环糊精上的羧基能和银纳米粒子形成很好的Ag-COO- 相互作用,更有利于稳定银纳米粒子[15]。
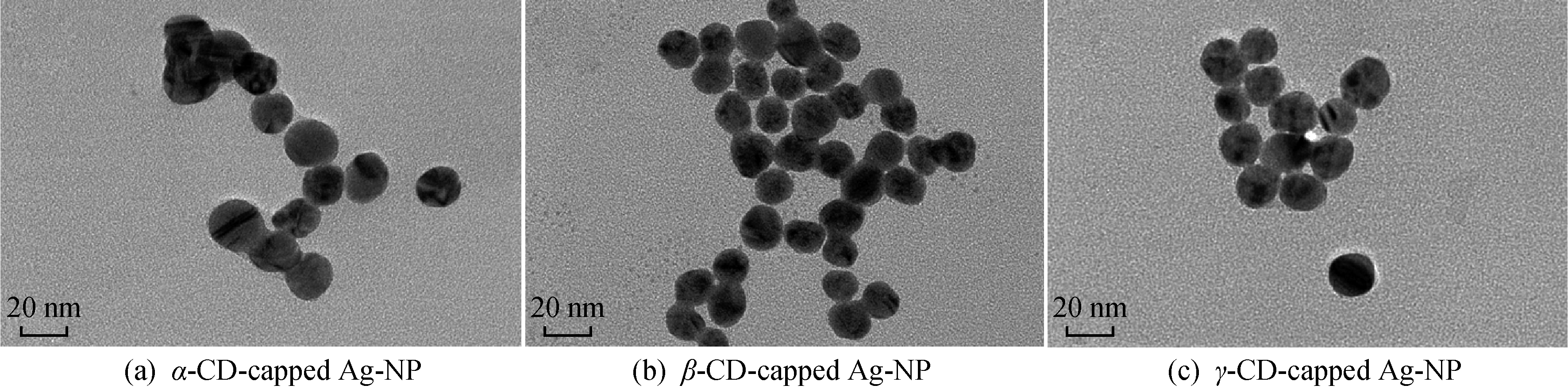
图5 不同环糊精包覆的银纳米粒子的TEM图
Fig.5 TEM images of different CD-capped Ag-NPs
不同环糊精原位还原法制得的银纳米粒子的TEM如图5所示,从图中可以看到,环糊精包覆的银纳米粒子的粒径很均一,用Nano Measure软件统计得到α/β/γ-环糊精包覆的银纳米粒子的粒径分别为 18.25、16.46、18.54 nm。
2.4 催化性能
对硝基苯酚的还原反应是检验纳米金属催化效率的模型反应[16]。取3 mL 已经配制好的对硝基苯酚(4-NP)和硼氢化钠(NaBH4)的混合溶液(c4-NP=0.1 mmol/L; cNaBH4=20 mmol/L)于比色皿中,加入银纳米粒子催化剂,混合均匀后,立即用UV-vis 分光光度计在250~500 nm的波段每隔2 min 扫描一次。如图6所示,加入银纳米粒子催化剂后,400 nm处对硝基苯酚钠的紫外吸收峰随着反应的进行逐渐降低,300 nm处对氨基苯酚钠的紫外吸收峰逐渐升高,280 nm处和310 nm处为对硝基苯酚还原反应的2个等吸光度点(Isosbestic Point)。
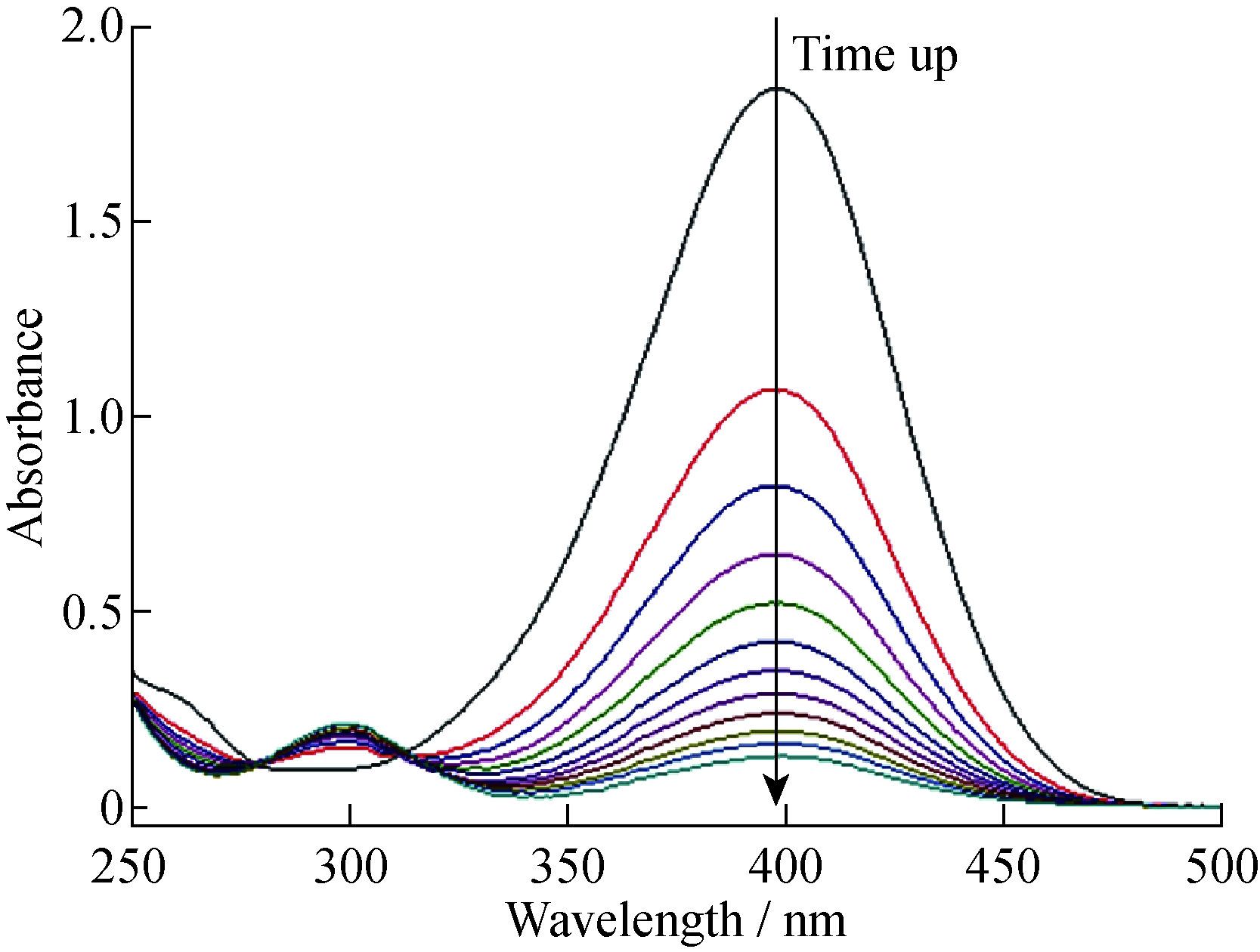
图6 银纳米粒子催化对硝基苯酚还原反应的UV-vis吸收光谱随时间的变化(T=293.15 K)
Fig.6 Time dependence of UV-vis spectra for the reduction of 4-NP by NaBH4 in the presence of Ag-NP at 293.15 K
在对硝基苯酚的还原实验中,硼氢化钠是大大过量的,因此,在整个反应过程中,可以将硼氢化钠的浓度看成常数,此时该反应便可以看作是“拟一级”反应,反应动力学方程见式(1):
(1)
式中:ct是t时刻4-NP的浓度;kapp是催化剂表观反应速率;S是单位体积催化剂表面积;k是单位催化面积的催化反应速率。
测试不同环糊精制备得到的银纳米粒子在不同温度(288.15、293.15、298.15、303.15、308.15 K)下催化对硝基苯酚的反应动力学,得到不同温度下的催化反应速率k,如图7所示。
根据阿仑尼乌斯方程:
k=Ae-Ea/RT
(2)
式中:R是理想气体常数;T是反应温度。表观活化能Ea可以从ln k和T-1的线性拟合中求得。从图7可以看到,β-环糊精包覆的银纳米粒子催化对硝基苯酚的活化能最小,α-环糊精包覆的银纳米粒子的活化能最大;从催化速率来看,γ-环糊精和β-环糊精包覆的银纳米粒子的催化活性明显高于α-环糊精包覆的银纳米粒子,这可能是由于β-环糊精和γ-环糊精的空腔较大,更有利于反应底物对硝基苯酚钠通过环糊精(稳定剂)接触到纳米金属表面。
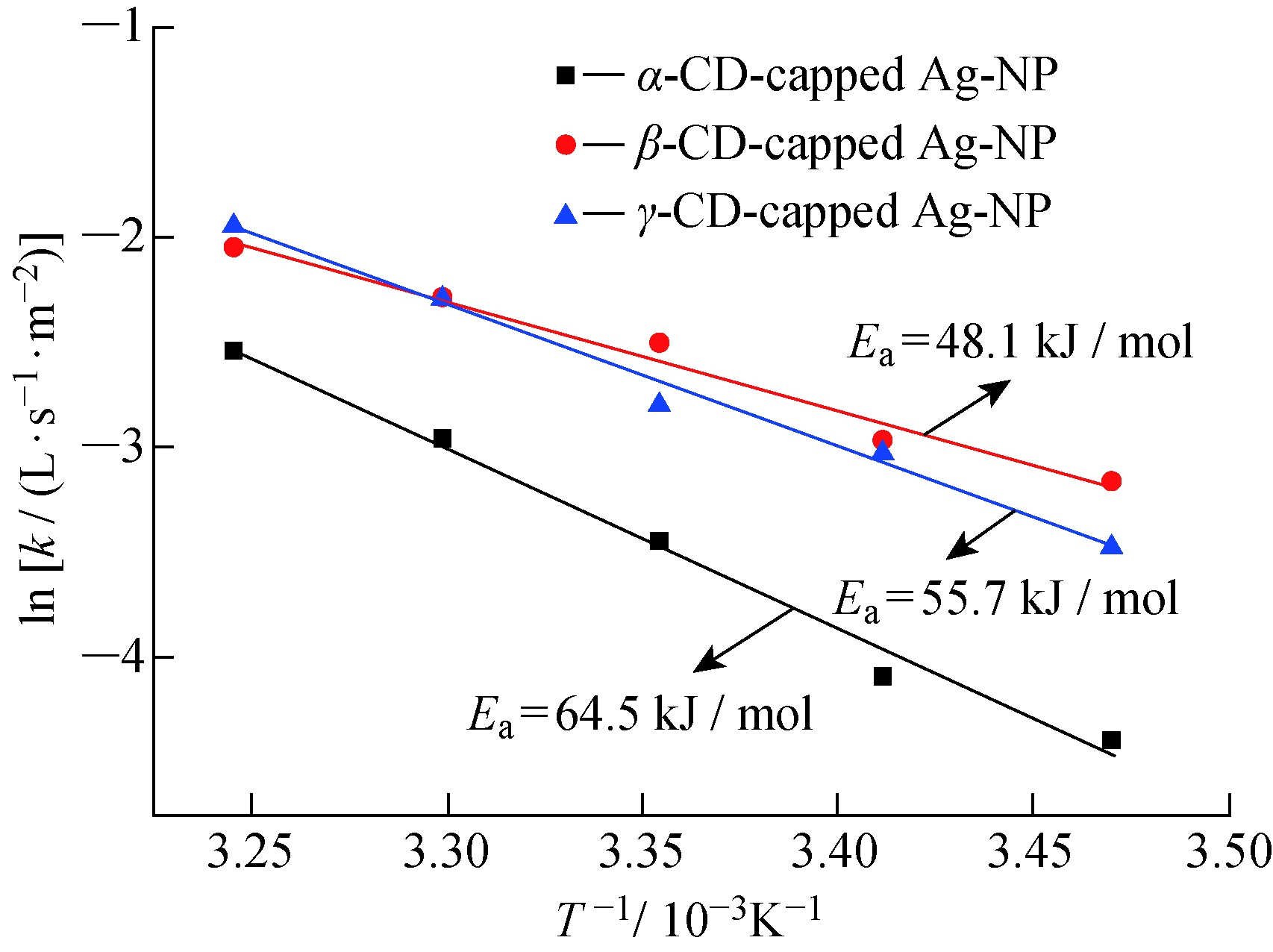
图7 不同环糊精包覆的银纳米粒子的ln k与T-1 的关系
Fig.7 Plot of ln k versus T-1 with different CD-capped Ag-NPs
3 结 论
本文利用环糊精作为还原剂和稳定剂,在碱性水溶液中制备得到窄粒径分布的银纳米粒子,并研究了pH和环糊精浓度对银纳米粒子粒径的影响,发现碱性太强和环糊精浓度太低会导致银纳米粒子团聚。在催化对硝基苯酚的实验中发现,β-环糊精包覆的银纳米粒子催化对硝基苯酚的活化能最小,并且β-环糊精和γ-环糊精包覆的银纳米粒子的催化活性明显高于α-环糊精,这可能是与环糊精的包合作用和空腔尺寸有关。
参考文献:
[1] BURDA C, CHEN X D, NARAYANAN R, et al. Chemistry and properties of nanocrystals of different shapes[J]. Cheminform, 2005, 36(27): 1025-1102.
[2] LIU J J, WANG J, ZHU Z M, et al. Cooperative catalytic activity of cyclodextrin and Ag nanoparticles immobilized on spherical polyelectrolyte brushes[J]. AIChE Journal, 2014, 60(6): 1977-1982.
[3] LIU X C, XU Y, WANG X H, et al. Stable and efficient loading of silver nanoparticles in spherical polyelectrolyte brushes and the antibacterial effects[J]. Colloids and Surfaces B: Biointerfaces, 2015, 127: 148-154.
[4] WANJALA B N, FANG B, LUO J, et al. Correlation between atomic coordination structure and enhanced electrocatalytic activity for trimetallic alloy catalysts[J]. Journal of the American Chemical Society, 2011, 133(32): 12714-12727.
[5] LU Y, MEI Y, DRECHSLER M, et al. Ag nanocomposite particles: Preparation, characterization and application[J]. Macromolecular Symposia, 2007, 254: 97-102.
[6] SCOTT R W, WILSON O M, CROOKS R M. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles[J]. Journal of Physical Chemistry B, 2005, 109(2): 692-704.
[7] WANG M, WANG J, WANG Y M, et al. Synergetic catalytic effect of α-Cyclodextrin on silver nanoparticles loaded in thermosensitive hydrogel[J]. Colloid and Polymer Science, 2016, 294(6): 1087-1095.
[8] SHARMA G, BALLAUFF M. Cationic spherical polyelectrolyte brushes as nanoreactors for the generation of gold particles[J]. Macromolecular Rapid Communications, 2004, 25(4): 547-552.
[9] LU Y, MEI Y, DRECHSLER Markus, et al. Thermosensitive core-shell particles as carriers for Ag nanoparticles: Modulating the catalytic activity by a phase transition in networks[J]. Angewandte Chemie-International Edition, 2006, 45(5): 813-816.
[10] ARMELAO L, BOTTARO G, CAMPOSTRINI R, et al. Synthesis and structural evolution of mesoporous silica silver nanocomposites[J]. Nanotechnology, 2007, 18(15): 625-637.
[11] GUPTA P, BAJPAI M, BAJPAI S K. Investigation of antibacterial properties of silver nanoparticle-loaded poly (acrylamide-co-itaconic acid)-grafted cotton fabric[J]. Journal of Cotton Science, 2008, 12(3): 280-286.
[12] RAVEENDRAN P, FU J, WALLEN S L. Completely “green” synthesis and stabilization of metal nanoparticles[J]. Journal of the American Chemical Society, 2003, 125(46): 13940-13941.
[13] LIU J, QIN G W, RAVEENDRAN P, et al. Facile “green” synthesis, characterization, and catalytic function of β-D-glucose-stabilized Au nanocrystals[J]. Chemistry: A European Journal, 2006, 12(8): 2131-2138.
[14] 张震, 谭连江, 龚兵, 等. 基于“分子胶”的新型两亲性嵌段共聚物β-CD-PLA的合成及其胶束化[J]. 功能高分子学报, 2017, 30(3): 321-326.
[15] HUANG T, MENG F, QI L M. Facile synthesis and one-dimensional assembly of cyclodextrin-capped gold nanoparticles and their applications in catalysis and surface-enhanced raman scattering[J]. The Journal of Physical Chemistry C, 2009, 113(31): 13636-13642.
[16] HERVES P, PEREZ-LORENZO M, LIZ-MARZAN L M, et al. Catalysis by metallic nanoparticles in aqueous solution: Model reactions[J]. Chemical Society Reviews, 2012, 41(17): 5577-5587.